SARS-CoV-2 variant hits again! Hybribio's coronavirus detection kit effectively covers Omicron (B.1.1.529), helping control the spread of novel coronavirus
The recent rebound of the domestic epidemic has caused much concern. Worse still, there is even a heavy bombshell abroad - WHO has officially included the B.1.1.529 variant of SARS-Cov-2 in the VOC (Variant of Concern) list, named Omicron.

WHO officially named the B.1.1.529 variant as Omicron
Where did this variant come from?
The Omicron variant was first reported to the WHO from South Africa on November 24, 2021. The epidemiological situation in South Africa is characterized by three distinct peaks in reported cases, the latest of which was dominated by the Delta variant. In recent weeks, there has been a dramatic increase in infection, coinciding with the detection of the Omicron variant. The first known confirmed Omicron infection came from a specimen collected on November 9, 2021.
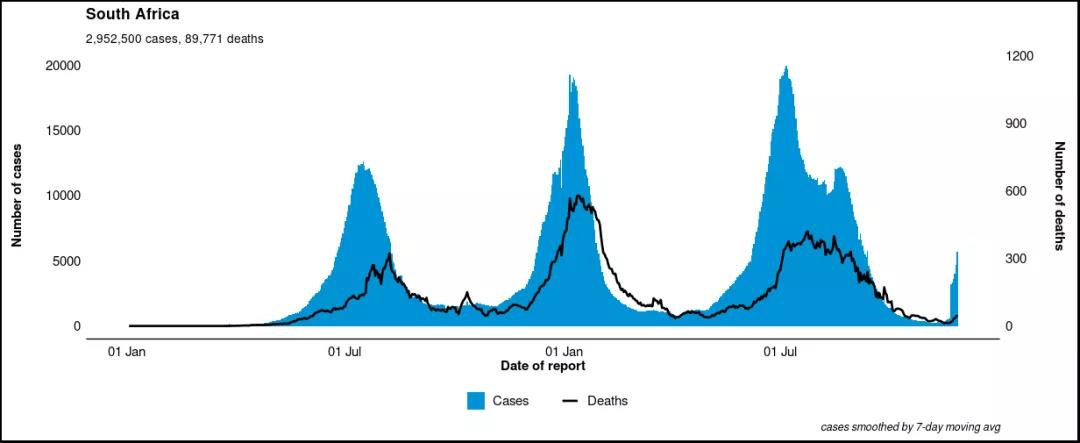
The epidemiological characteristics of the South African outbreak indicate severe rebound of the epidemic in recent weeks
Omicron variant appeared in China
In addition to the rapid rebound of the outbreak in South Africa, the Omicron variant has also appeared in China (Hong Kong). On October 22, 2021, a 36-year-old man in Hong Kong tested negative on a PCR and then flew to South Africa on October 23. On November 11, the man took Qatar Airways flight QR818 (via Doha) to return to Hong Kong, being tested negative on a PCR after arriving at the airport. On November 12, the man tested positive on a subsequent PCR (asymptomatic) while in quarantine at the hotel; on November 15, the Hong Kong Department of Health (DH) announced that the man was infected with the "N501Y variant" (actually Omicron). The sequencing records of this strain were uploaded to the GISAID database on November 22.
After confirmation by international experts, it was found that in addition to N501Y, this variant has several threatening amino acid mutations, including mutations in the RBD and NTD structural domains of the stinging protein (immune escape, resistance to neutralizing antibodies), the combination of stinging protein H655Y+N679K+P681H (a triple mutation in the Flavin protease cleavage site that enhances infectivity), 105/107 deletion mutation in the NSP6 non-structural proteins (similar to Alpha/Beta/Gamma/Lambda, possibly enhancing immune escape), and the nucleocapsid protein R203K+G204R mutation (similar to Alpha, Gamma, Lambda, enhancing infectivity). Combined with the information of other virus strains found in South Africa, Pango Team officially named it as novel variant B.1.1.529.
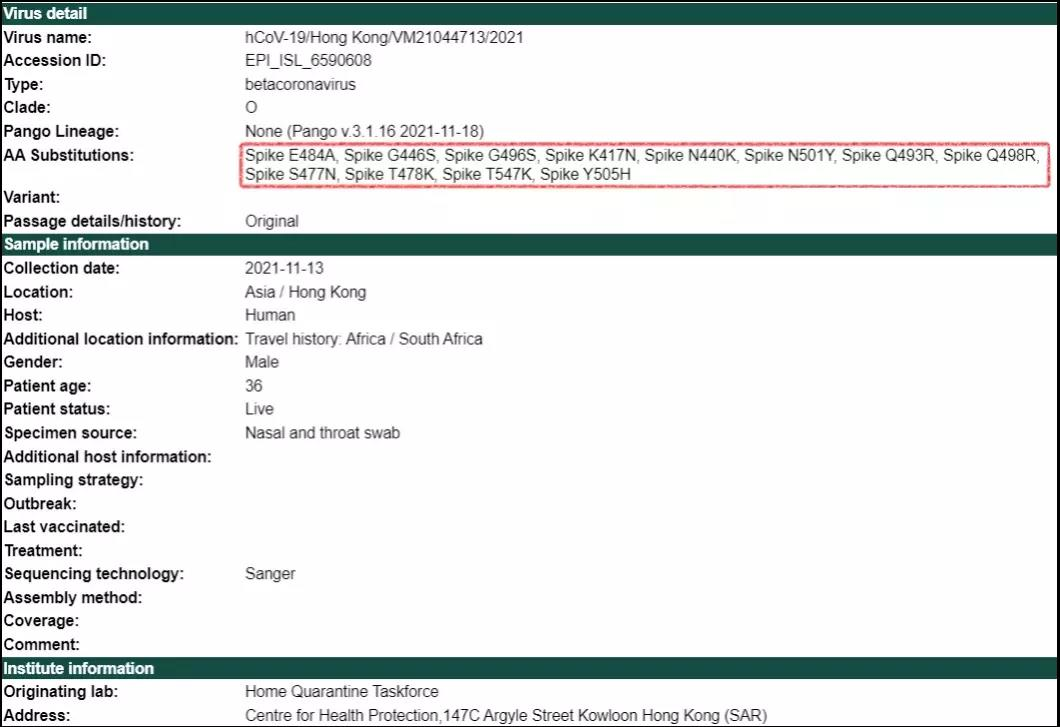
Sequencing information of the variant found in Hong Kong
Don't panic! Hybribio's coronavirus detection reagents effectively cover the types of known variants!
Hybribio’s registered 2019-nCoV Real-time PCR Kit (GXZZ 20213400269) can effectively cover the variants, including B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), Fin-796H, B.1.525 (Eta), N.9, B.1.616, B.1.617.1 (Kappa), B.1.617.2 (Delta), B.1.526 (Iota), B.1.427/B.1.429, C.37 (Lambda), B.1.621+B.1.621.1 (Mu), P.3 (Theta), B.1.640, B.1.1.529 (Omicron) and other variants. Most importantly, it is validated that the new variant B.1.1.529 (Omicron) is within the detection scope of the kit!
The features of the 2019-nCoV Real-time PCR Kit includes endogenous RNA control, intron-spanning primers & probes, effective quality control of sampling, preservation solution, extraction and amplification. Being tested with the national sensitivity references, the kit met the corresponding regulations. More specifically, S1~S5 can be detected as positive and the concentration of S5 is 412 copies /mL. Based on the market demand, we submitted an application for updates of packaging specification of COVID-19 detection kit to the National Medical Products Administration on May 20, 2021, simultaneously changing the product expiration date, sample storage conditions, increasing the transportation method and changing the minimum detection limit. Besides, on November 4, 2021 we obtained the updated registration certificate [GXZZ 20213400269]: product specifications 24 kits / box, 48 kits / box, 96 kits / box, 144 kits / box, the minimum detection limit is not higher than 450 copies / mL.


In addition, Hybribio's Novel Coronavirus (2019-nCoV) nucleic acid Detection Kit (Sanger sequencing method) was awarded the national invention patent (patent number: 202010460533) and was selected as an emergency research project by the Guangzhou Municipal Science and Technology Bureau. The kit can detect specific regions covering ORF1a, ORF1ab and 4 structural proteins including S, M, E and N genes. A Novel Coronavirus (SARS-CoV-2) Spike Gene Mutation Detection Kit (Nested PCR + Sanger Sequencing) has been developed for variants with the S gene mutation hotspot region, which has obtained the national invention patent (Patent No. ZL 2021 1 0514745.X) and EU CE certification (CMC/CE/2020/27052021.3). Moreover, through further improvement, it can now cover the full-length sequence of the S gene, and can detect both variants as well as differential diagnosis of new variant B.1.1.529 and non-mutant variants.
Hybribio Group has accumulated mature experience in fighting against the epidemic through rounds of epidemic prevention and control screening in many places, and from the production of novel coronavirus reagents, consumables and instruments to testing technicians and operation management. Under the leadership of local governments and relevant departments, Hybribio will continue to fight together with medical workers on the front line, provide protection for the lives and health of people nationwide, and fully cooperate with the government to fight the war against the novel coronavirus and build a healthy China!
