It’s the First! Hybribio Medical Lab’s COVID-19 Nucleic Acid Testing Accredited by ISO15189 Laboratory
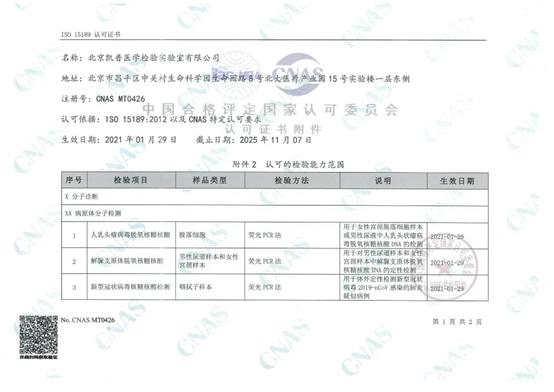
Recently, Beijing Hybribio Medical Laboratory’s COVID-19 nucleic acid testing and other expanded items have been awarded the ISO15189 accredition by the China National Accreditation Service for Conformity Assessment (CNAS), becoming the first independent clinical laboratory (ICL) that has passed ISO15189 accreditation for the COVID-19 nucleic acid testing project in China.

In November, CNAS appointed experts from the evaluation team to Beijing Hybribio Medical Laboratory for on-site evaluation and testing of its COVID-19 nucleic acid testing and other expanded items. The experts strictly followed the requirements of the ISO15189 international quality system index. Through methods such as listening to reports, on-site inspections, and consulting materials, the experts strictly reviewed and recorded management elements including quality management system, document control, service agreement, non-conformance identification and control, risk assessment, internal audit, and management review, as well as technical elements including personnel management, equipment and reagents, quality assurance, report and release of results, and information management, etc.
After three days of on-site review, the expert group agreed that Beijing Hybribio Medical Laboratory’s COVID-19 nucleic acid testing and other expanded items met the requirements of the "Medical Laboratory Quality and Competence Accreditation Guidelines" in terms of quality management system and technical capabilities. It was announced on November16th, 2020 that it passed the review successfully.

Early discovery, early detection and early diagnosis are the key steps of the prevention and control of the COVID-19 pandemic. With the pandemic sweeping across the world on a large scale, the technology of nucleic acid testing has been able to detect the coronavirus in the first time, which is of great significance for blocking the spread of the disease, controlling the spread of the pandemic and early intervention treatment. As the recognized "golden standard" of COVID-19 diagnosis, it is also an effective detection technology for clinical applications. The settings of laboratory, personnel, process, verification and other requirements have an important impact on the accuracy of detection.
As a professional, efficient and high-quality testing service provider, Hybribio Medical Laboratory Group including the Hong Kong Molecular Pathology Diagnostic Centre, Beijing Hybribio Medical Laboratory and Guangzhou Hybribio Medical Laboratory have all successively obtained ISO15189 laboratory accreditation and implemented the system to all of its medical labs. Over the years, Hybribio has actively participated in the national and provincial inter-laboratory quality assessment, and all the results have been completely passed, with the accuracy and stability of its test results recognized by authorities. Hybribio Medical Laboratory Group will continue to require itself with high quality and high standards, implement all testing items in accordance with national standards, strictly control the testing quality of each specimen, and provide clinical and patients with more scientific and accurate testing basis.