New Product Launch - Hybribio MTHFR Gene MCA Real-time PCR Kit Approved by NMPA
In August 17, 2023, Hybribio Biotech’s independently developed MTHFR Gene MCA Real-time PCR Kit (fluorescence PCR melting curve method) was officially awarded a Class III medical device registration certificate (registration number: GXZZ 20233401162) by the National Medical Products Administration (NMPA).
This kit detects the genetic polymorphism of the key enzyme in the folic acid metabolism pathway - 5,10- methylenetetra hydrofolate reductase (MTHFR) c.677C>T site in human venous whole blood samples, thereby assisting doctors in assessing the risk of patients’ folic acid metabolism ability, and then guiding the rational adjustment of folic acid supplementation dose to reduce the harm caused by insufficient or excessive folic acid supplementation.
Folic acid is a water-soluble vitamin, also known as vitamin B9, which is an essential element for nucleic acid and protein synthesis, and is necessary for cell growth and tissue repair. It is also an indispensable nutrient during embryonic development.
Assistance for diagnosis of H-type hypertension, providing precise treatment for patients
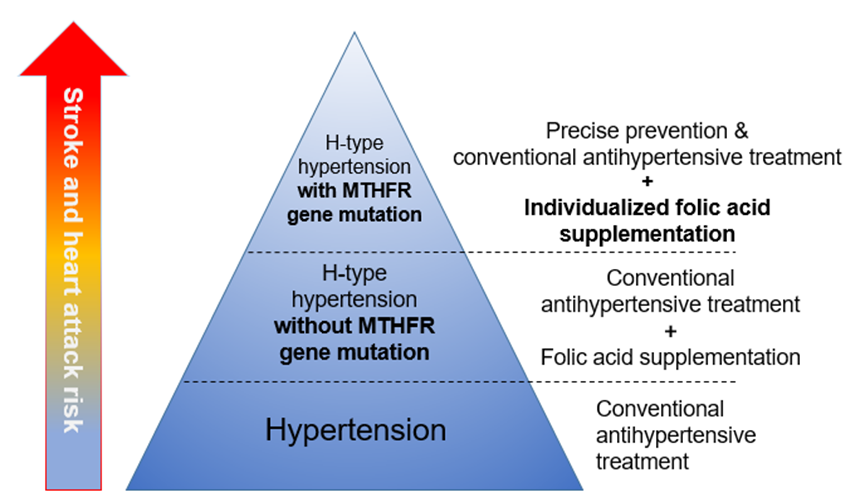
Hypertension is the main cause of death from cardiovascular and cerebrovascular diseases today. The “Report on the Nutrition and Chronic Disease Status of Chinese Residents (2020)” shows that the prevalence of hypertension among people aged 18 and above in China is as high as 27.9%. At present, there are about 245 million hypertensive patients, among which H-type hypertension accounts for 75% of all hypertensive patients. H-type hypertension is related to folic acid metabolism disorders, and MTHFR gene C677T mutation is the main genetic factor leading to increased homocysteine (Hcy) levels in the Chinese population. The “Expert Consensus on the Diagnosis and Treatment of H-type Hypertension” clearly states that MTHFR 677TT genotype is an independent risk factor for coronary heart disease and stroke. H-type hypertensive patients should undergo genotyping to determine the precise risk stratification of H-type hypertension. [4] By clarifying the patient’s MTHFR genotype, it can be determined whether MTHFR gene mutations affect the utilization rate of folic acid, leading to increased Hcy levels and increased disease risk. Many authoritative consensuses and expert guidelines, such as the “Guidelines for Rational Use of Hypertension Drugs”, recommend MTHFR genotyping to clarify the cause of disease and help doctors perform risk stratification and individualized treatment according to different patient genotypes, effectively preventing hypertension complications and reducing the risk of cardiovascular and cerebrovascular diseases.
Personalized Folic Acid Supplementation to Prevent Birth Defects
The "Guideline for Folic Acid Supplementation during Periconceptional Period to Prevent Neural Tube Defects (2017)" points out that the gene mutation of the key enzyme MTHFR in the folic acid metabolism pathway affects the absorption and metabolism of folic acid. The TT homozygous mutation at the MTHFR 677 site and low blood folic acid concentration are related to increased blood homocysteine concentration, and high homocysteine blood levels increase the risk of neural tube defects and other adverse pregnancy outcomes [1].
The "Multidisciplinary Expert Consensus on Rational Folic Acid Supplementation in China (2020)" points out that more than 50% of newborn neural tube defects (NTDs) cases are related to insufficient folic acid in early pregnancy, and folic acid supplementation can significantly reduce the incidence of NTDs. Long-term folic acid supplementation also helps reduce the risk of cardiovascular and cerebrovascular diseases. However, long-term high-dose (>1mg/d) folic acid supplementation (including folic acid fortified foods) may pose health risks, such as increasing the risk of certain cancers (such as colorectal cancer, prostate cancer), exacerbating neurodegenerative diseases, etc. [2]
Therefore, it is particularly important to adopt a personalized folic acid supplementation plan based on the MTHFR gene polymorphism of each expectant mother and father. The Maternal and Child Health Center of the Chinese Center for Disease Control and Prevention has included folic acid utilization ability gene detection and risk assessment in clinical application guidelines, recommending that expectant mothers undergo folic acid utilization ability gene detection before supplementing with folic acid, and choose a personalized supplementation plan based on risk stratification. [3]

The Hybribio MTHFR Gene MCA Real-time PCR Kit uses the fluorescence PCR melting curve method, which can distinguish wild-type, homozygous mutant, and heterozygous mutant at one detection site with one detection channel. It can detect all sites in the same reaction tube, supports automatic interpretation, and is convenient and fast.
MTHFR Gene MCA Real-time PCR Kit (Fluorescence PCR Melting Curve Method)
Intended Use:
The kit detects MTHFR c.677C>T gene polymorphism, providing auxiliary diagnosis for patients with coronary atherosclerotic heart disease, hypertension, heart failure, angina pectoris, hyperhomocysteinemia and other related cardiovascular diseases; guides folic acid medication, assesses the risk of patients' folic acid metabolism ability, and reduces the harm caused by folic acid deficiency or excess.
Target population group:
(1) Patients with coronary atherosclerotic heart disease, hypertension, heart failure, angina pectoris, hyperhomocysteinemia and other related cardiovascular diseases to reduce the risk of related diseases.
(2) Mostly used for expectant couples and pregnant women to assist in personalized folic acid supplementation.
(3) Pregnant women with preeclampsia or corresponding risk signs.
Applicable Instruments:
Hongshi SLAN-96S, Roche Light Cycler 480 II.
References:
[1] Periconceptional Folic Acid Supplementation to Prevent Neural Tube Defects Guideline Working Group. Guideline for Periconceptional Folic Acid Supplementation to Prevent Neural Tube Defects (2017) [J]. Chinese Journal of Reproductive Health, 2017, 28(5): 401-410.
[2] Multidisciplinary Expert Consensus on Rational Folic Acid Supplementation in China [J]. Medical Guide, 2021, 40(01): 1-19.
[3] "Compilation of Clinical Genetic Testing Projects for Maternal and Child Health Care" Volume Six Clinical Application Guidelines Twenty-One "Folic Acid Utilization Ability"
[4] Li Jianping, Lu Xinzheng, Huo Yong et al. Expert Consensus on the Diagnosis and Treatment of H-type Hypertension [J]. Chinese Journal of Hypertension.2016.