Brazil Central Public Health Laboratory: Hybribio’s COVID-19 Real-time PCR Kit brings fast and accurate test results
With the onset of the novel coronavirus, the demand for COVID-19 diagnostic kits is soaring, creating a huge shortage. This shortage of COVID-19 diagnostic kits under the current situation has severely hindered the Brazil government’s effort to control the epidemic. Thus, the Bahia State Government in Brazil went to great lengths to purchasing 60,000 tests of Hybribio’s COVID-19 diagnostic kit via a local enterprise. The first batch of Hybribio's kits has been delivered to Brazil at the end of March. However, according to Brazil's BNews, the Governor of Bahia, Rui Costa had to arrange a special plane to pick up the reagents due to consecutive flight cancellations.
Veja, a Brazilian magazine, reported on 19 April that Hybribo’s diagnostic kit has gone through several tests in Brazil Central Public Health Laboratory, and the results of which have proved that within just 90 minutes, the diagnostic kit can produce testing results with an accuracy of more than 99%. Folha De S.Paulo, another Brazilian media, reported that Hybribio’s diagnostic kit detects the existence of novel coronavirus by marking the ORFlab genes and N genes of the virus through the collected nasopharyngeal cells. Compared with the other three types of COVID-19 diagnostic kits, Hybribio’s kit is able to achieve an accuracy of 100% and has acquired verifications in all the tests using positive and negative samples.

Image Source:Folha De S.Paulo
Renata Cassia Pires, associate professor at the Department of Clinical Analysis at the Federal University of Rio Grande do Norte and researcher at Fiocruz, says that one of the advantages of this kit is that it can generate results very fast.
“The average time for performing the test under laboratory condition is less than two hours. This is because the kit performs the amplification and the detection of the genetic material at the same time. Hybribio’s kit marks two targeting genes and thus ensures the high sensitivity and high accuracy, which provides a higher level of precision”, he adds.
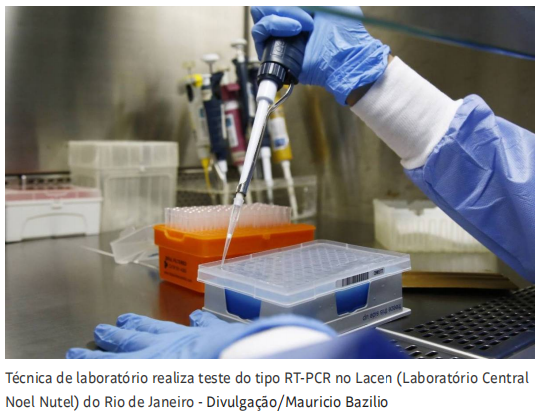
Image Source:Folha De S.Paulo
A note in the Bahia State government document states that the RT-PCR diagnostic reagents are allocated only to the Bahia Central Public Health Laboratories. Only these laboratories are capable of conducting molecular diagnostic tests. The results of molecular diagnostic tests are the gold standards because this kind of tests can detect the genome of the virus.
According to these reports, the implementation of the tests will be easy and smooth because Brazil already has the PCR technology for diagnoses and the essential equipment for running the tests.
In early April, Hybribio obtained the import license and the market approval issued by Anvisa. Currently, the coronavirus outbreak has been alleviated slightly in China. However, here in Hybribio, we do not slack off. While we are striving to consolidate the results of fighting the coronavirus domestically, we are also helping other countries. To meet the growing demand for COVID-19 diagnostic kits in the global market, we have made efforts to increase our production. This is a dark hour in human history, but a bright prospect awaits so long as the world unite and fight the virus as a Community of Shared Future for Mankind. Being a part of this Community, Hybribio is willing to cooperate with other countries, presenting to the world that China is a reliable country.