Full marks! Hybribio medical laboratories for novel coronavirus nucleic acid detection nationwide haven passed the external quality assessment
Recently, the Shanghai Clinical Inspection Center (CNAS PT0025) and the Shanghai Clinical Center for Quality Control issued a notice on the implementation of a plan, in which the 2019-nCoV nucleic acid detection capability could be verified. Laboratories which meet the biosafety requirements and are qualified to carry out genetic testing experiments, are eligible for the assessment. Beijing, Shanghai, Guangzhou, Jinan, and many other Hybribio medical laboratories have all passed the external quality assessment, which indicated that Hybribio medical laboratories had established a network for novel coronavirus detection. In this battle against the epidemic in China, Hybribio’s medically professional team played an indispensable role in protecting the health of our people!

EQA (external quality assessment) is a process in which multiple laboratories analyze the same specimen and the external independent authority collects and reports the results reported by the laboratories, so as to evaluate the operations of the laboratory. The comparison between laboratories determines the laboratory's ability to calibrate, test, and monitor its sustainability. EQA is a kind of verification activity to ensure that the laboratory maintains a high level of testing capability.
The verification of capability in novel coronavirus nucleic acid detection based on the assessment criteria that the results are 100% compliant, otherwise it is judged as unqualified. For qualified laboratories, Shanghai Clinical Inspection Center will issue a certificate. Faced with such strict requirements, many Hybribio medical laboratories located in Beijing, Shanghai, Guangzhou, Nanchang, Jinan, Chengdu, Taiyuan, Kunming, Fuzhou, etc. have all completed and passed this external quality assessment. This not only demonstrated the strict specifications of Hybribio in laboratory biosafety equipment, personnel biosafety training and management procedures, but also reflected Hybribio Medical Inspection Center's attitude towards improving both hardware and software, and its determination to fight against the "epidemic" .
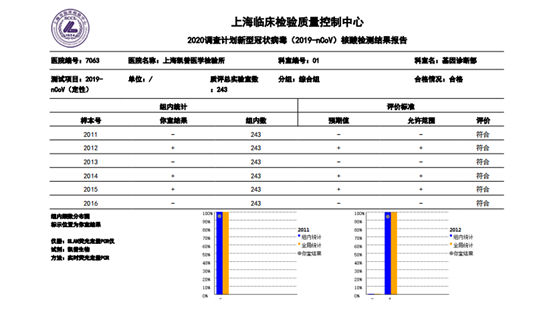
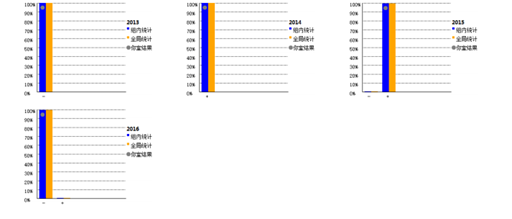

Up to now, Hybribio’s detection network for novel coronavirus has been deployed in major cities across the country. Hybribio has the technical strength to carry out detection for large enterprises and universities. Hybribio medical laboratories of Wuhan, Guangzhou, Beijing, Chongqing, Xi'an, Nanchang, Jinan and so on have been included in the list of third-party institutions that can carry out the novel coronavirus nucleic acid detection. "The pursuit of the country is the pursuit of Hybribio". As a national enterprise, Hybribio responded to the country's call for "Strengthen the economic epidemic prevention", fully supported the inspection of the epidemic situation for companies which are back in production, and worked hard to prevent and control the novel coronavirus, escorting the life and health of the people.
